
The Basics of Nanoparticles
Health risks associated with nanoparticles has limited their incorporation in cosmetics yet their outstanding performance has led to a rise in popularity in utilizing these materials.
In today’s highly competitive beauty industry, brands are making sure that they can provide the most effective and efficient services and products to their clients. Product innovation is key in this whole process. In the search for a skin whitening agent, innovators have found that rucinol is more effective than hydroquinone, kojic acid, and arbutin; and that it has a lower irritation rate. However, the product stability could still be improved to be better. Introducing Natori Lumisome RC, with improved rucinol formulation to produce more effective results, in a form of safe and stable product.
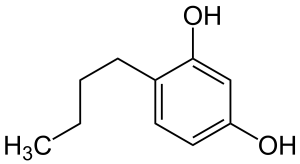
Rucinol, or 4-n-butylresorcinol, is a resorcinol derivate which is used as skin whitening ingredients. It is a highly effective whitening and depigmentation agent with an excellent safety profile. The first efficacy of rucinol was reported in 1995, and its efficacy and safety had been studied ever since. Compared to other well-known whitening agents like kojic acid, hydroquinone, and arbutin, rucinol is proven to be more effective and safer in treating hyperpigmentation problems in skin. Safety factor becomes extremely important aspect for a whitening actives since they may cause side effects such as irritation or redness.
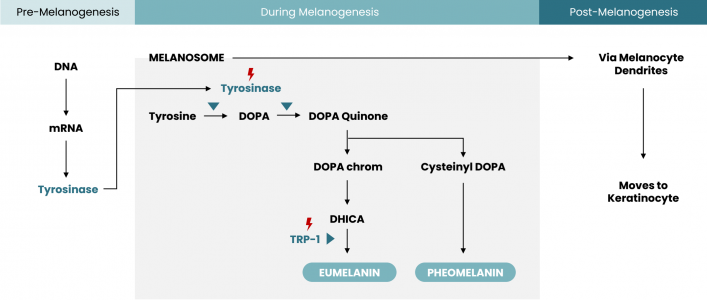
In melanogenesis, pigments are derived from a common precursor from l-tyrosine through tyrosinase process. There is also an enzyme that played a part in producing eumelanin pigments, called tyrosinase-related protein-1 (TRP-1). Rucinol works by inhibiting the activity of both tyrosinase and TRP-1, thus prevents eumelanin production.
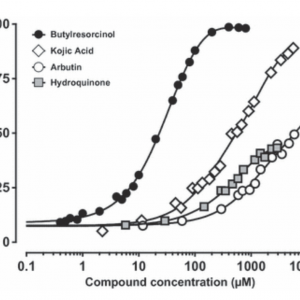
The L-DOPA oxidase activity of tyrosinase was determined at various concentrations of the inhibitors to allow for the calculation of IC50 values. The data represent the mean of three independent experiments.
4-butylresorcinol proved to be a highly effective inhibitor of human tyrosinase with an IC50 of 21 μmol/L and complete enzyme inhibition at concentrations above 100 μmol/L.
Kojic acid was more than 20 times less potent with an IC50 at 500 μmol/L and maximum inhibition (89%) at 5.6 mmol/L concentration. Meanwhile arbutin and hydroquinone are poor inhibitors of human tyrosinase with IC50 values in the millimolar range, i.e. approximately 6500 μmol/L for arbutin and 4400 μmol/L for hydroquinone. Neither arbutin nor hydroquinone completely inhibited human tyrosinase.
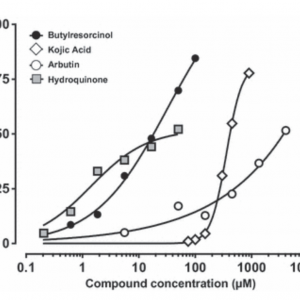
Melanin content of skin models was determined after 13 days of cultivation in the presence of various inhibitor concentrations. The data represent the mean of five independent experiments.
4-butylresorcinol was the most potent inhibitor with an IC50 of 13.5 μmol/L. It could be observed that 4-butylresorcinol is more effective than hydroquinone at concentration higher than 20 μmol/L. Hydroquinone inhibited melanin production with an IC50 below 40 μmol/L.
Arbutin showed only marginal efficacy on melanin with an IC50 for inhibition of > 5000 μmol/L. Kojic acid inhibited melanin synthesis with an IC50 > 400 μmol/L with a surprisingly steep curve. Concentration below 200 μmol/L only marginally inhibited melanin production, 5% inhibition at 150 μmol/L.
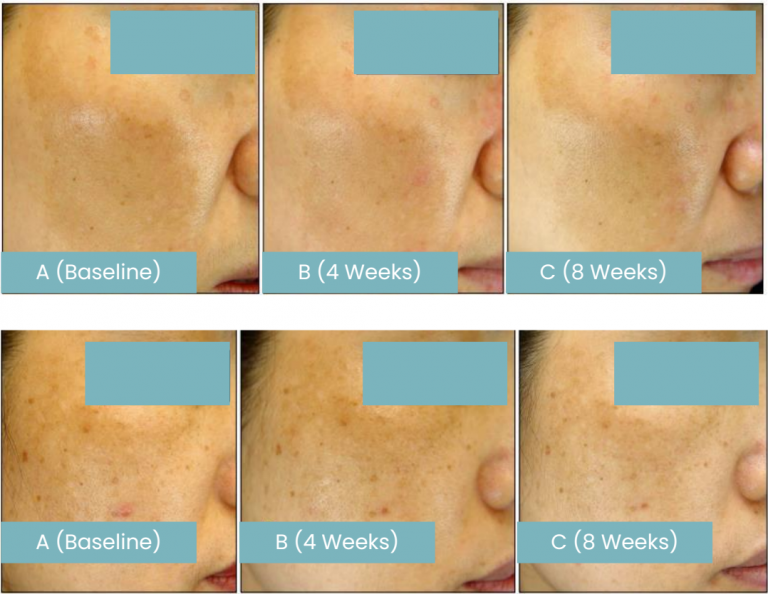
The test was conducted on twenty patients with melasma, a skin condition that shows symmetric distribution of hyperpigmentation on sun-exposed areas, which is predominantly observed on the face. After 8-week study period, melanin index (MI) of the patients drops 4.87%, showing improvement on melasma.
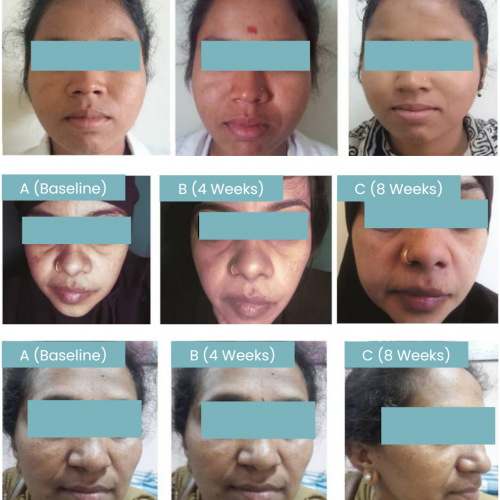
The test was conducted on fifty-two subjects with melasma, treated using 0.3% rucinol cream to observe the efficacy and safety of the product. The parameter used in the assessment was modified Melasma Area Severity Index (mMASI) score, observed after 8-week application. It was found that the mMASI score decrease from 14.9 to 6.9 on women in average, and from 13.4 to 6.4 on men in average. There was no adverse effect reported nor observed during the research. Therefore, it is concluded that 0.3% rucinol cream is safe and effective for treating melasma.
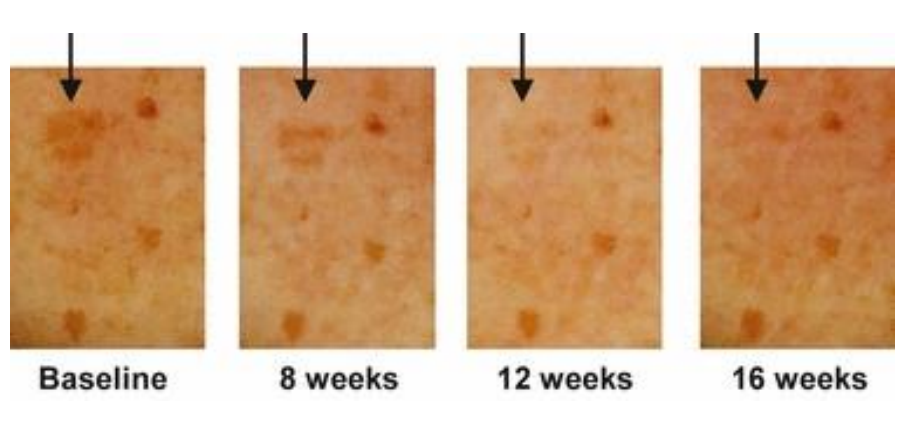
After 4 weeks of treatment, the treated spots were observed to be lighter than the control spots. Improvement continued over the entire treatment period, and after 16 weeks some of the spots were undistinguishable from the surrounding skin. After treatment, the age spots were monitored for several weeks. Even after 4 weeks without treatment, the age spots previously treated with 4-butylresorcinol were still significantly lighter than the vehicle-treated spots.
Natori Lumisome RC from Ecoori is an improved version of rucinol for skin whitening. It is water soluble and could be easily applied to a wide range of personal care products due to its water-based property. Additionally, it has improved stability, safety, and efficacy compared to the regular rucinol.
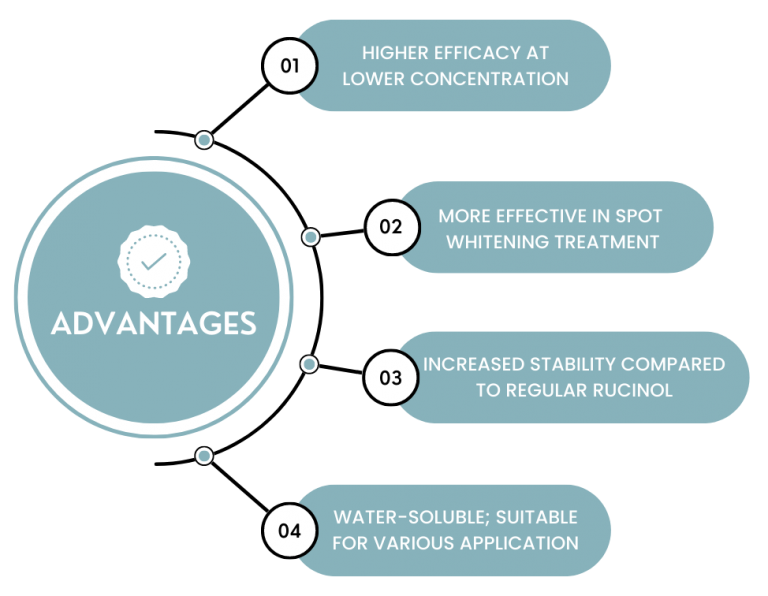
The advantages of Natori Lumisome RC are summarized as follows:
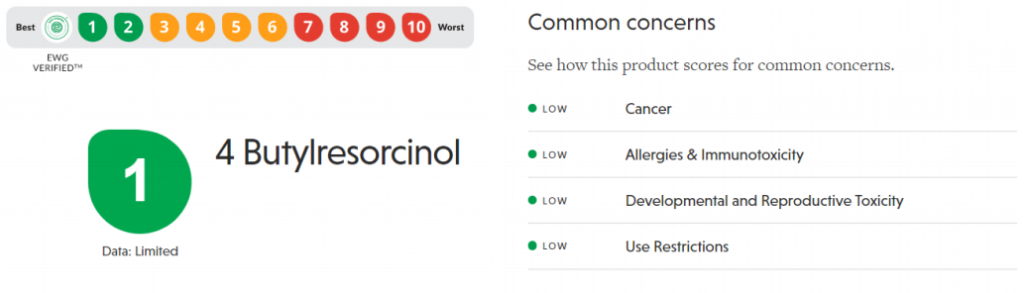
Referring to EWG rating for ingredients, rucinol is considered very safe with 1 EWG rating. The scores are low for all the common concerns for cancer, allergies and immunotoxicity, developmental and reproductive toxicity, and with low use restrictions as well.
A study conducted to review the clinical use of depigmenting agents also showed that rucinol had the potential to act as whitening ingredients with less irritation compared to other ingredients such as hydroquinone, tretinoin, triple-combination cream (TCC), arbutin, azelaic acid, and kojic acid.
References:
The Efficacy and Safety of 4 n butylresorcinol 0.1% Cream for the Treatment of Melasma: A Randomized Controlled Split face Trial , Ann Dermatol Vol. 22, No. 1, 2010
Assessment of efficacy, safety, and tolerability of 4 n butylresorcinol 0.3% cream: an Indian multicentric study on melasma: Clinical, Cosmetic and Investigational Dermatology 2016:9
4-n-butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation, JEADV2013,27 (Suppl.1),19–23
https://www.ewg.org/skindeep/ingredients/716217-4BUTYLRESORCINOL/
Current clinical use of depigmenting agents, Jung Won Shin and Kyoung Chan Park, 2014.

Health risks associated with nanoparticles has limited their incorporation in cosmetics yet their outstanding performance has led to a rise in popularity in utilizing these materials.

How is green surfactant is being manufactured? Our principal manufactures amino acid-based surfactant with amidation process in aqueous solution. Find out more about this now from here.

Common Problems of Vitamin C for Skin Care. Vitamin C is already well-known for its beneficial effects on our body. Hence, there have been many attempts to include this into skin care…
Copyright © 2023 Maha Chemicals (Asia) Pte Ltd